Excelya helps minimize your time to market, so you can reach as many people as possible with your intervention.
The COVID-19 pandemic showed the world how important rapid scientific response is in halting infectious disease outbreaks.
Infectious diseases, caused by microorganisms like viruses, bacteria and fungi, remain major causes of death globally. According to the World Health Organization (WHO), lower respiratory infections, diarrheal diseases, tuberculosis, and HIV/AIDS were among the 20 leading causes of death in 2019.
Whether an epidemic is ongoing or emerging, we understand that rapid response makes a difference. At Excelya, we have the experience and expertise to make sure your clinical trial runs smoothly so you can progress to market without delay.

Whether your clinical trial relates to a viral, bacterial or fungal infectious disease, we have the expertise to support you. Excelya has a network of sites and investigators, including key opinion leaders (KOLs) and Therapeutic Advisors in each field.
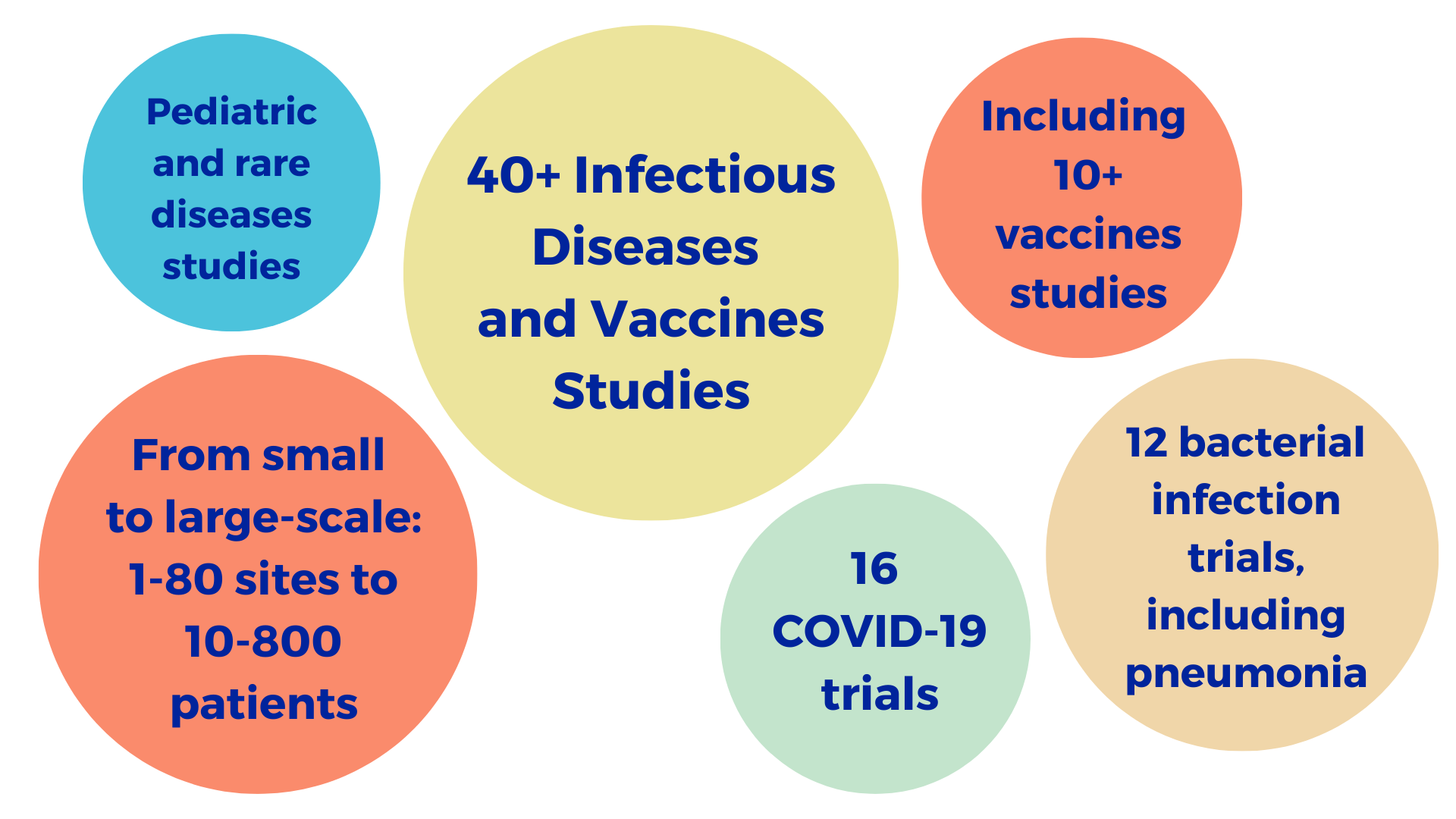
With experience of over 40 infectious disease and vaccine studies, including pediatric and rare disease, we have worked on viral, bacterial and fungal infectious disease clinical trials ranging from early phase to post-market studies.
Our most recent viral pandemic has changed the way we live, and it had a significant impact on clinical trials. Over the three-year course of the pandemic, there were more than 676 million cases of COVID-19, resulting in more than 6.8 million deaths. The global research response prevented millions more deaths by developing treatments and vaccines, with over 13 billion vaccine doses delivered around the world.
Excelya was proud to support this effort: we took part in 16 studies related to COVID-19. This enhanced our viral infectious disease experience, which covers HIV, influenza, hepatitis C and D, rhinovirus and more. With extensive hands-on expertise in infectious disease drug and treatment development, Excelya has a unique advantage in this area.
As a treatment for bacterial infectious diseases, antibiotics have extended the average human lifespan by 23 years. However, the rise of antimicrobial resistance (AMR) is a global challenge that requires solutions – including new antibiotic candidates. Excelya’s experts have worked on 18 bacterial and fungal clinical trials, giving us broad experience we can apply in every phase.
Vaccines are key solutions to many infectious diseases, and clinical trials can vary greatly depending on the trial type, study design, and type of vaccine being tested. Our experts have experience with a broad range of vaccine trials, including prophylactic and adjuvant, helping you navigate the nuances.
Our global talent pool of 900+ experts have extensive experience with infectious disease and vaccine clinical trials in all phases. In the first three years of this decade, we managed 42 infectious disease trials, including:
This broad experience means we have encountered the roadblocks you’re likely to face. Excelya Study Project Managers, scientific and medical experts know how to smooth out your journey.

With our collaborative one-team approach, we combine our expertise with your in-depth knowledge of the disease area, maximizing your chances of success. Whatever your support needs, our experts will strive for excellence, delivering high-quality, reliable work that accelerates your progress.
Put people first. Our people-centric approach means participants come first, improving your recruitment and retention results. And the collaborative links we build with patient associations, clinics and more can help you succeed.
Apply our experience. When we work with you on your infectious disease trial, we apply our experience across dozens of similar trials to optimize your processes and improve efficiency from trial design to data analysis.
Exceed your targets. When we work together, we take on your goals – and we share responsibility for achieving them.
Whether you’re conducting first in-human trials for a vaccine or working on post marketing safety surveillance for a established treatment, or anywhere in between, we can support you.
With full-service support from trial design through to data analysis, or more targeted support for regulatory submission or medical writing, Excelya has the right option to suit your needs – and our talent pool of over 900 experts are ready to help.
