Get inspection-ready with eTMF: With Excelya’s dedicated eTMF team, you can make sure your trial master file is inspection ready and secure.
Data conformance issues can set your trial back, costing you time and money – this is a critical issue for almost one-third of FDA submissions.
Digitalization will help you avoid quality issues: with an electronic trial master file (eTMF) – a digital version of the trial master file (TMF) –you become inspection ready and increase the safety of your documentation.
With the secure cloud-based platform Veeva Vault, your study team can unify documentation, access study data and avoid errors. And using its password-protected environment helps you ensure data privacy for your participants.
At Excelya, our highly specialized team of dedicated eTMF experts apply their extensive life science experience to make sure your eTMF management is smooth, helping accelerate your trial.

Documenting your clinical trial is an essential step in the process, particularly for compliance. With an eTMF, you use hardware and software to collect, store and manage essential documents and data throughout the lifespan of your trial.
When you work with Excelya, you access the market-leading eTMF software Veeva Vault – a cloud-based content management platform and suite of applications that gives you a single source of truth, helping you reduce complexity and increase business agility. Veeva Vault is always accessible, ensuring your trial is ready for inspection at any time.
Our dedicated eTMF team has extensive experience with Veeva Vault. We use best-in-class processes, procedures to help you understand your study holistically and run it efficiently.
Putting people first in your clinical trial means protecting their privacy and safety. An eTMF can support this by securing your trial data safely in a password-protected environment. Excelya’s eTMF management prioritizes digital safety and protection.
Data encryption diminishes many of the data safety vulnerabilities related to a paper TMF system. With Veeva Vault, you can specify which users have access to which information, ensuring people only work with directly relevant data. Additional precautions, such as regular password changes and operational system checks, mean you can rest assured that your data is safe.
Our approach to eTMF management also reduces related risks. The eTMF makes regulatory access easier, so the auditing process is more efficient, reducing business risks. And as it’s cloud-based, your eTMF is less susceptible to environmental risks and vulnerabilities such as fire or elemental damage, theft and negligence.
When you work with Excelya for your eTMF management, you get support from our dedicated team, whose focus is delivering eTMF projects – a unique offering in the CRO industry.
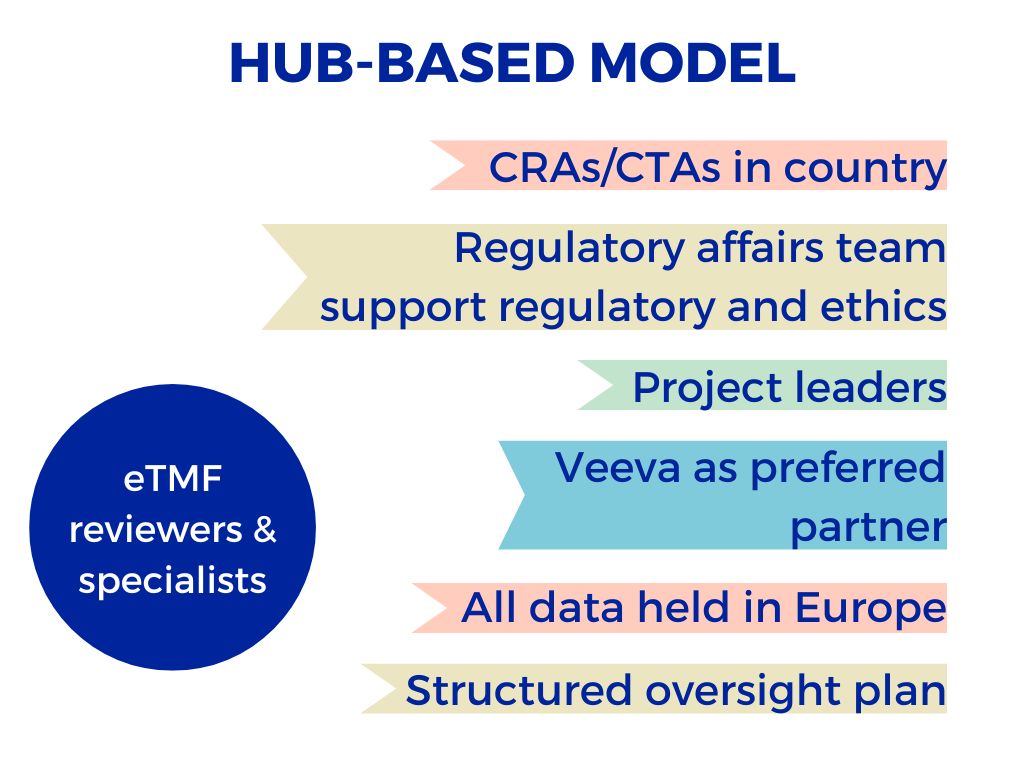
Excelya’s hub-based model provides trained and certified eTMF specialists who are highly knowledgeable about processing across a range of therapeutic areas, and passionate about the positive impact eTMF technology can ultimately have on patients’ lives.
They support your trial by combining theory and practice: they are highly qualified, with a master’s or PhD-level education and three years of clinical trials experience on average. The team also remains at the forefront of innovation, propelled by regular training.


Choose from our full-service offer or stand-alone services
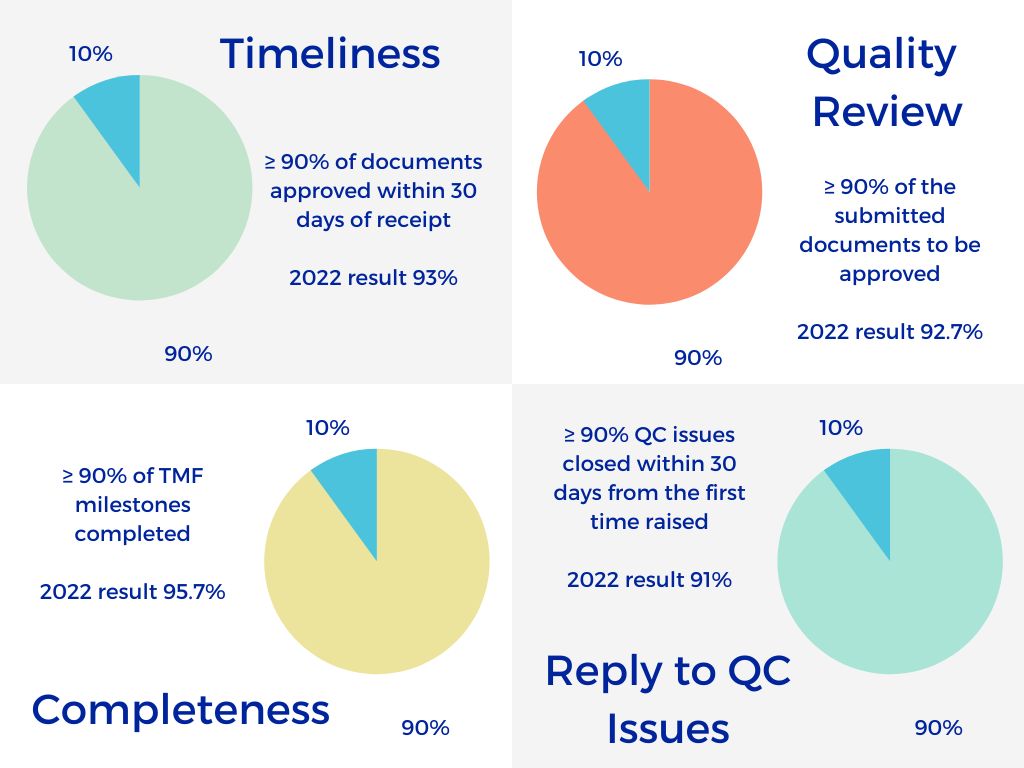
To be inspection-ready, your Trial Master File needs to be complete, consistent and accurate. We create a TMF plan, upload and review your documents to ensure that they are inspection ready, and then archive them using Veeva’s eTMF software to make sure your documents are readily available when you need them.
When you decide to secure and accelerate your trial with an eTMF, you can choose the level of support you need from Excelya. We can take care of everything, end-to-end, or just one aspect of your eTMF management.
Whatever support you choose, we can deliver:
When you work with Excelya on your eTMF, you can also access our expert Statistics team. With error-free and unbiased statistics, you can draw conclusions and support decision making. Our statistics experts work across the trial design process, data monitoring, analyses, closing phase and reporting.
Discover how Excelya can supercharge your statistics.
DEDICATED,
FULL-TIME eTMF TEAM
Uniquely specialized team whose focus is solely on delivering eTMF projects
AVERAGE 2 YEARS OF eTMF-SPECIFIC EXPERIENCE
A high level of task-related expertise, with up to 30 years of clinical experience
EXCEPTIONALLY QUALIFIED TEAM MEMBERS
Our eTMF experts have a Master/PhD level education and receive regular training

HIGHLY KNOWLEDGEABLE STAFF
Experience in clinical studies across therapeutic areas and phases