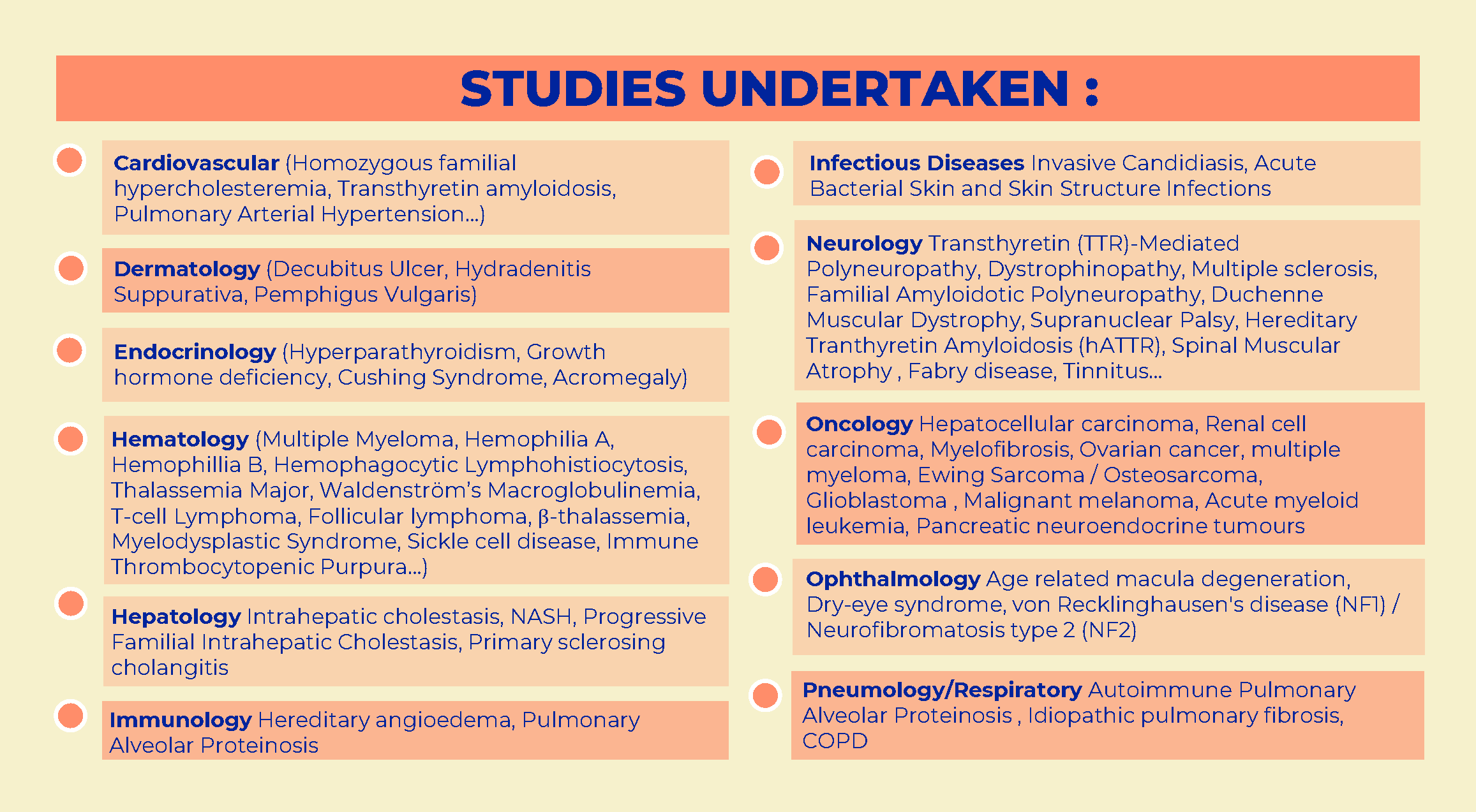
Rare and pediatric disease trials come with complex challenges that require specific expertise and experience. We have the people to help you solve them.
By definition, rare or orphan diseases affect very few people, but together, they have a significant impact: According to rarediseaseday.org there are 7,000 identified rare diseases which affect an estimated 30 million people in Europe alone.
This poses some unique challenges for clinical trial sponsors: how can you recruit the participants to run a successful trial when there are so few patients with each disease? And which sites would be best to select?
Many rare diseases affect children, and pediatric trials bring additional challenges. They require specific protocols, involve additional regulations and demand expertise and experience.
Excelya can share that to support your trial. Our global team of experts have worked on a range of rare disease and pediatric trials, and we understand the needs of these patients as well as the challenges you face in running a trial.

Read our Case Study which demonstrates how Excelya took advantage of our global reach in Central Eastern Europe to rescue the recruitment of a rare disease study.
Read our Case Study which demonstrates how Excelya succeeded in recruiting in a rare Post-AMM project with a very narrow population.
Each patient is unique, with their own individual journey, challenges and needs. These people are at the heart of your healthcare innovation, and we’ll always put them first to help you succeed.
Our first step is always understanding the patients you want to help – especially for rare diseases and trials involving children. To understand, we must listen, hear, and acknowledge the challenges, complexities, and limitations that people face.
With a global talent pool of experts, we have worked on over 130 rare or orphan disease trials and pediatric trials. Our knowledge isn’t just theoretical: we have encountered the challenges you face and worked out how to overcome them.
We have a Rare Disease & Pediatrics MD as our Therapeutic Area expert and Board-certified Pediatricians on staff.
Over 50% of our project management and clinical teams have rare disease and pediatric experience.


Did you know?
• A disease is rare when it affects fewer than 1 in 2,000 people.
• 70% of rare diseases start in childhood.
• 300 million people worldwide are suffering with a rare disease.
• Almost 1 out of 5 cancers is rare.
Our extensive experience in rare and pediatric disease trials has led us to develop tried and tested approaches to everything from patient recruitment to biological follow-up.
We work with patient associations. When your patient pool is small, it’s important to have open, two-way engagement with the right people. By establishing authentic relationships with patient associations, we make sure we can help you meet their needs.
We leverage our global reach. We have established relationships with study sites and institutions around the world, giving you a head start.
We apply our expertise and experience. We know about the diseases you work with, and we integrate that knowledge with our hands-on experience in the field to avoid obstacles and reduce risk.
We strive for excellence. We care about your goals and the patients you want to help, and we share responsibility for the outcomes of your trial. Our people are committed to delivering high-quality, reliable work that accelerates your progress.
We can support your trial with:
• Patient centricity – we always put people first
• Patient recruitment – we understand their needs as well as yours
• Guaranteed secure pathology diagnosis – we have rigorous quality controls in place
• Biological follow-up – our experts manage the process
• Biostatistics and biomarker expertise – we can help you with the specifics